Revolutionizing Cardiac Care: The Tiny, Dissolvable Pacemaker
In a groundbreaking leap for medical technology, researchers at Northwestern University have unveiled what is claimed to be the world’s smallest pacemaker—a device so tiny it can fit inside the tip of a syringe, be injected into the body, and dissolve harmlessly once its work is done.
4/20/20258 min read
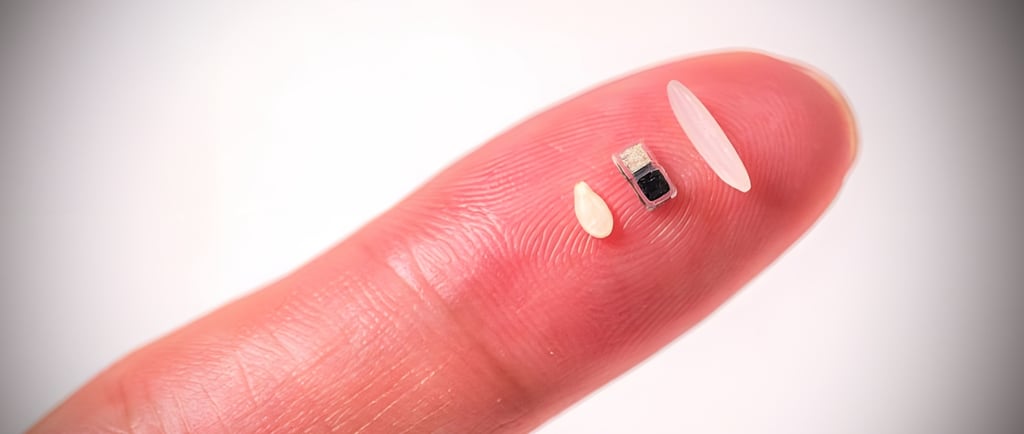
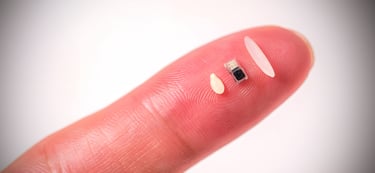
In a groundbreaking leap for medical technology, researchers at Northwestern University have unveiled what is claimed to be the world’s smallest pacemaker—a device so tiny it can fit inside the tip of a syringe, be injected into the body, and dissolve harmlessly once its work is done. This innovative pacemaker, smaller than a grain of rice, operates without wires or traditional batteries, relying instead on light-based activation and a galvanic cell powered by the body’s own fluids. Designed primarily for newborns with congenital heart defects, this bioresorbable, wireless device promises to transform cardiac care by offering a minimally invasive, temporary pacing solution that eliminates the need for follow-up surgeries. But how is this miniature marvel manufactured, and what cutting-edge technologies make it possible? This article delves into the intricate details of its production, the revolutionary technologies involved, and the implications for the future of bioelectronics, while also addressing the question of trust in a device engineered to disappear.
The Need for a Tiny Pacemaker
Congenital heart defects affect approximately 1% of newborns worldwide, with around 40,000 babies in the U.S. alone born with such conditions each year. Many of these infants require temporary pacing after corrective heart surgeries to maintain a stable heart rhythm while their hearts heal, typically for about seven days. Traditional temporary pacemakers, however, pose significant challenges, particularly for pediatric patients. These devices often involve wires sewn onto the heart, connected to external power sources, which can lead to complications such as bleeding, infection, or heart muscle damage during removal—a risk tragically highlighted by the death of astronaut Neil Armstrong in 2012 due to complications from wire removal.
For newborns, whose hearts are as small and fragile as a walnut, the size and invasiveness of conventional pacemakers are particularly problematic. The need for a smaller, less invasive solution drove Northwestern University’s team, led by bioelectronics pioneer John A. Rogers and experimental cardiologist Igor Efimov, to develop a pacemaker that could be injected non-invasively, function wirelessly, and dissolve naturally, reducing the physical and emotional burden on patients and their families.
Manufacturing the World’s Smallest Pacemaker
Creating a pacemaker smaller than a grain of rice—approximately 1 millimeter in scale—requires precision engineering, advanced materials science, and innovative manufacturing techniques. The device, detailed in a study published in Nature on April 2, 2025, is a culmination of years of research at Northwestern’s Querrey Simpson Institute for Bioelectronics. Below, we explore the key steps and technologies involved in its production.
1. Material Selection: Biocompatible and Bioresorbable Components
The pacemaker’s ability to dissolve safely in the body is central to its design. To achieve this, every component is made from biocompatible and bioresorbable materials that break down into non-toxic byproducts when exposed to bodily fluids. Key materials include:
Magnesium (Mg) and Molybdenum (Mo): These metals serve as electrodes in the galvanic cell, the device’s power source. When in contact with biofluids (such as blood or interstitial fluid), these metals form a simple battery that converts chemical energy into electrical energy to power the pacemaker.
Silicon (Si): A phototransistor made from silicon responds to infrared light pulses, enabling wireless control. Silicon is chosen for its biocompatibility and ability to degrade safely over time.
Polymer Encapsulation: The device is encased in a thin, flexible polymer layer, such as poly(lactic-co-glycolic acid) (PLGA), which controls the rate of dissolution. By varying the polymer’s composition and thickness, researchers can program the device to function for a specific period—typically seven days—before dissolving completely.
These materials are selected not only for their functional properties but also for their ability to dissolve into biocompatible end products, ensuring no harmful residues remain in the body.
2. Microfabrication Techniques
Manufacturing a device at the millimeter scale requires microfabrication techniques typically used in the semiconductor industry. The process involves:
Photolithography: This technique uses light to pattern thin films of materials onto a substrate, creating the intricate structures of the pacemaker, such as the phototransistor and electrodes. Photolithography allows for nanoscale precision, critical for a device of this size.
Thin-Film Deposition: Layers of magnesium, molybdenum, and silicon are deposited onto a substrate using techniques like physical vapor deposition (PVD) or chemical vapor deposition (CVD). These methods ensure uniform, ultra-thin layers that maintain the device’s compact profile.
Etching and Patterning: Selective etching removes unwanted material, shaping the electrodes and phototransistor. Wet or dry etching processes are used to achieve the precise geometries needed for the device’s functionality.
These processes are conducted in cleanroom environments to prevent contamination, ensuring the device meets stringent medical safety standards.
3. Assembly and Encapsulation
Once the individual components are fabricated, they are assembled into a cohesive device. This involves:
Integration of the Galvanic Cell: The magnesium and molybdenum electrodes are positioned to interact with biofluids, forming the galvanic cell. A tiny light-activated switch, the silicon phototransistor, is integrated to control the device’s on/off state.
Polymer Coating: The assembled device is encapsulated in a bioresorbable polymer layer using spin-coating or dip-coating techniques. This coating not only protects the device during its functional period but also controls its dissolution rate.
Miniaturization Challenges: Shrinking the device to fit within a syringe tip required moving away from near-field communication (NFC) protocols, which relied on a bulky antenna. Instead, the team developed a light-based activation system using infrared pulses, significantly reducing the device’s footprint.
4. Testing and Validation
Before clinical use, the pacemaker undergoes rigorous testing to ensure safety and efficacy. The device has been tested in animal models (mice, rats, dogs) and ex vivo human and pig hearts, demonstrating its ability to maintain stable heart rhythms. The team is working toward U.S. Food and Drug Administration (FDA) approval for human clinical trials, with an estimated timeline of five years for market readiness. Northwestern has also founded NuSera Biosystems, a startup aimed at commercializing the technology.
Groundbreaking Technologies Involved
The tiny pacemaker is a triumph of interdisciplinary innovation, combining advances in materials science, bioelectronics, and optoelectronics. Below are the key technologies that make this device possible:
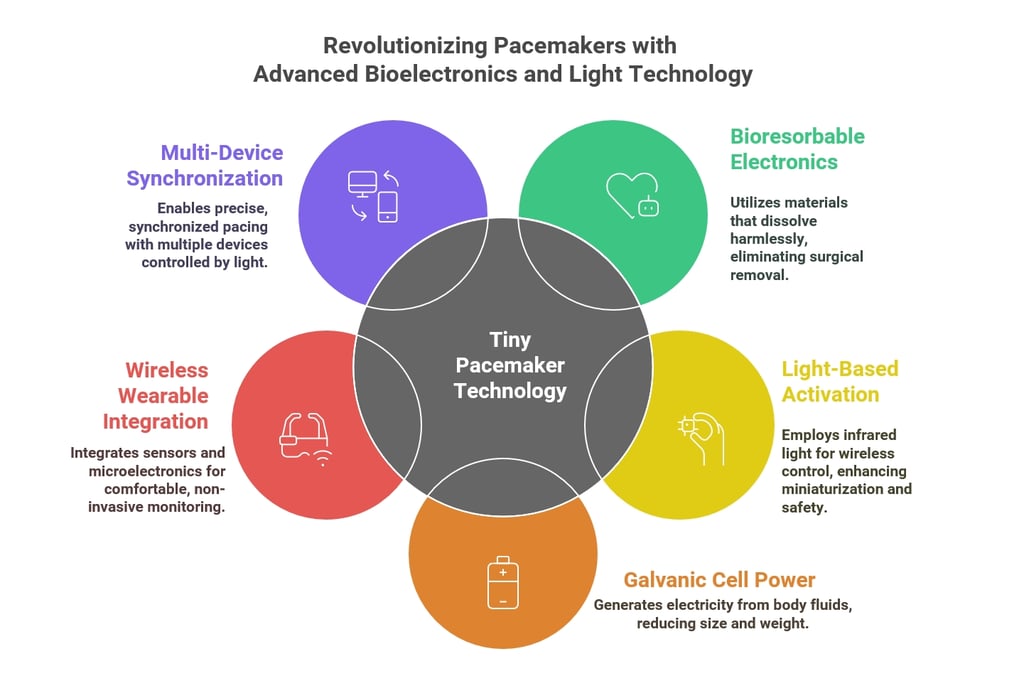
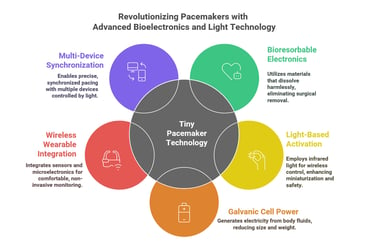
1. Bioresorbable Electronics
The pacemaker builds on Northwestern’s earlier work in bioresorbable electronics, notably a quarter-sized dissolvable pacemaker introduced in 2021. Bioresorbable electronics are designed to function for a predetermined period before dissolving harmlessly, eliminating the need for surgical removal. This technology relies on materials like magnesium, molybdenum, and PLGA, whose degradation rates can be precisely controlled. The 2025 pacemaker represents a significant leap in miniaturization, reducing the device’s size by over an order of magnitude while maintaining functionality.
2. Light-Based Activation (Optoelectronics)
Traditional pacemakers use radio frequency (RF) signals or NFC for wireless control, requiring antennas that limit miniaturization. The new pacemaker employs a light-based system, where a wearable patch on the patient’s chest emits infrared light pulses that penetrate skin, bone, and muscle to activate the silicon phototransistor. This optoelectronic approach offers several advantages:
Miniaturization: Eliminating the antenna reduces the device’s size dramatically.
Precision: Multiple pacemakers can be activated independently using different wavelengths of light, enabling synchronized pacing across the heart.
Safety: Infrared light is non-ionizing and safe for biological tissues, minimizing risks to patients.
3. Galvanic Cell Power Source
The pacemaker’s power source is a galvanic cell, a simple battery that generates electricity through a chemical reaction between two metal electrodes (magnesium and molybdenum) and the body’s biofluids, which act as an electrolyte. This eliminates the need for a traditional battery, further reducing size and weight. The galvanic cell produces a small but sufficient current to deliver electrical pulses to the heart, with the reaction products safely absorbed by the body.
4. Wireless Wearable Integration
The pacemaker pairs with a soft, flexible, wireless patch worn on the chest, which monitors heart rhythms and delivers light pulses when irregularities are detected. This wearable is lightweight and non-invasive, designed to be comfortable for patients, including newborns. The patch uses advanced sensors and microelectronics to ensure accurate detection and precise light delivery, showcasing the integration of wearable technology with implantable devices.
5. Multi-Device Synchronization
The pacemaker’s small size allows multiple devices to be implanted across the heart, each independently controlled by different light wavelengths. This capability enables precise, synchronized pacing to address complex arrhythmias or support recovery after procedures like transcatheter aortic valve replacements. This multi-device approach could revolutionize treatments for conditions requiring targeted electrical stimulation, such as atrial fibrillation.
Applications Beyond Cardiac Care
While the pacemaker was developed with pediatric cardiology in mind, its versatility extends to other areas of bioelectronic medicine. Potential applications include:
Nerve Stimulation: The technology could stimulate nerves to treat conditions like Parkinson’s disease or chronic pain.
Wound Healing and Bone Regeneration: Electrical stimulation from similar devices could accelerate tissue repair or bone growth.
Pain Management: By blocking pain signals, the technology could offer non-pharmacological pain relief.
The ability to implant multiple devices and control them independently opens new possibilities for personalized, targeted therapies, potentially transforming how we approach a wide range of medical conditions.
Trust in a Disappearing Device
The concept of a medical device designed to dissolve raises legitimate questions about reliability and safety. Can we trust a pacemaker that disappears? Several factors inspire confidence in this technology:
Extensive Testing: The device has been rigorously tested in animal models and ex vivo human hearts, with results published in a peer-reviewed journal (Nature). Ongoing efforts toward FDA approval will further validate its safety.
Biocompatible Materials: All components are made from materials with well-documented safety profiles, ensuring no toxic residues remain after dissolution.
Controlled Dissolution: The polymer encapsulation allows precise control over the device’s lifespan, ensuring it functions reliably for the required period (e.g., seven days) before dissolving.
Clinical Precedents: Northwestern’s earlier bioresorbable pacemaker, introduced in 2021, demonstrated the feasibility of dissolvable electronics in preclinical studies, paving the way for this smaller iteration.
However, challenges remain. The device is still years away from human use, with clinical trials needed to confirm its performance in live patients. Long-term effects of bioresorbable materials in diverse patient populations must also be studied. Additionally, the reliance on a wearable patch introduces potential risks, such as device malfunction or improper placement, which could affect performance. Addressing these concerns through robust clinical trials and quality control will be critical to building trust.
The Future of Bioelectronics
The tiny pacemaker exemplifies the potential of bioelectronics to address unmet medical needs with elegance and precision. Its development reflects a broader trend toward minimally invasive, patient-centered technologies that integrate seamlessly with the body. As John A. Rogers noted, “This technology opens up many possibilities… not only for heart conditions but also for healing nerves, bones, and even pain management”.
Looking ahead, the pacemaker could democratize access to advanced cardiac care, particularly in low-resource settings where surgical infrastructure is limited. Its syringe-based delivery and lack of need for removal surgery make it a cost-effective solution for temporary pacing. Furthermore, the technology’s adaptability suggests a future where bioresorbable devices are tailored to individual patients, offering personalized therapies with minimal risk.
Conclusion
Northwestern University’s tiny, dissolvable pacemaker is a testament to the power of interdisciplinary innovation, combining materials science, optoelectronics, and bioengineering to create a device that is as functional as it is fleeting. By leveraging biocompatible materials, microfabrication techniques, and light-based activation, the team has crafted a solution that addresses the unique needs of newborns with congenital heart defects while opening doors to broader applications in bioelectronic medicine. While questions about trust in a disappearing device are valid, the rigorous testing, proven materials, and clinical precedents provide a strong foundation for confidence. As this technology moves toward clinical trials and eventual market availability, it promises to redefine cardiac care—and perhaps medicine as a whole—by proving that even the smallest interventions can have a monumental impact.
References:
Zhang, Y., Rytkin, E., Zeng, L., et al. Millimetre-scale bioresorbable optoelectronic systems for electrotherapy. Nature. 2025;640(8057):77-86. doi: 10.1038/s41586-025-08726-4
Northwestern University. (2025, April 2). World’s smallest pacemaker is activated by light. ScienceDaily.
Additional sources as cited throughout the article.
